Mitochondrial Disease Therapeutic Pipeline
Several treatments for mitochondrial diseases are already making their way along this path. Now, mito patients and their families will be able to see the treatment approaches being developed, where they are in the development pipeline, and what mitochondrial diseases they target.
Conditions in the Pipeline
- Barth Syndrome
- KSS-CPEO
- Leigh Syndrome
- LHON
- MELAS
- MERRF
- Primary Mitochondrial Myopathies (PMM)
- MIDD
- Pearson Syndrome
- Pyruvate Dehydrogenase Complex Deficiency (PDCD)
- Seizures
- TK2 Deficiency
Pre-Clinical Development
Phase I
Phase II
Phase III
Regulatory Approval
Phase II/III tests how well a new treatment works and compares the new treatment with a standard treatment.
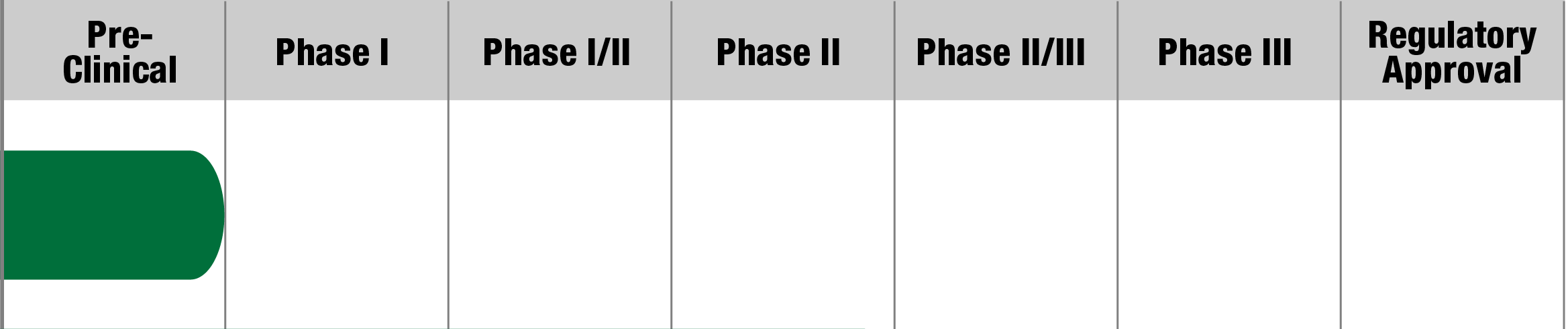
Barth Syndrome
Elamipretide (SS-31/MTP-131)
Stealth BioTherapeutics
Study Name:
TAZPOWER
Status:
Study Complete
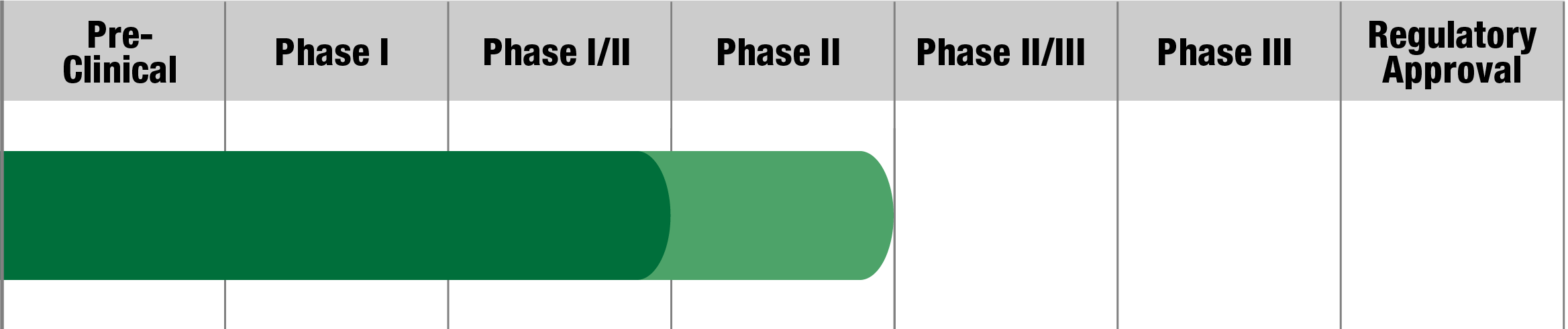
CoQ10 deficiency
BPM31510IV
BPG Bio, Inc.
Status:
Received Rare Pediatric Disease
designation from FDA
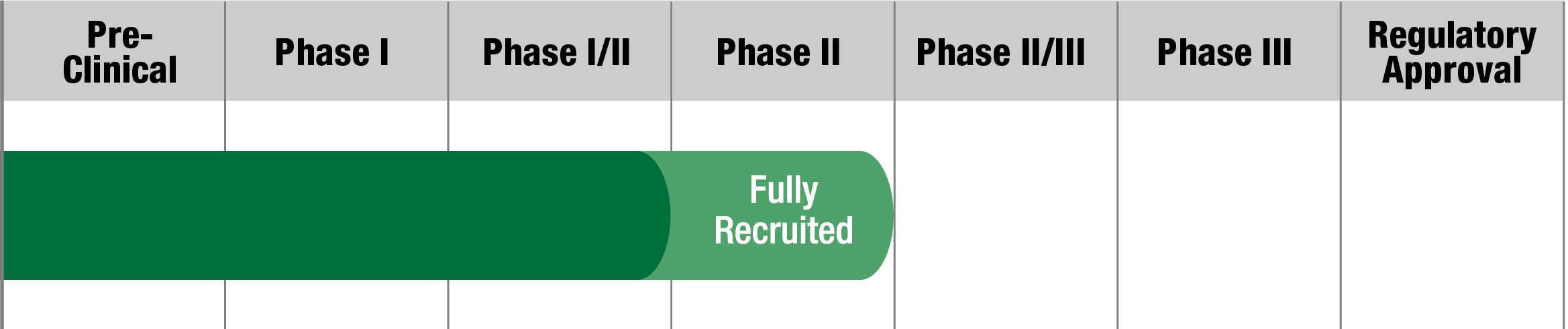
KSS-CPEO
KL1333
Abliva AB
Study Name:
FALCON
Status:
Fully Recruited
Leigh Syndrome
MNV-201
Minovia
Status:
Progressing to clinical development

NV354
Abliva AB
Status:
Progressing to clinical development
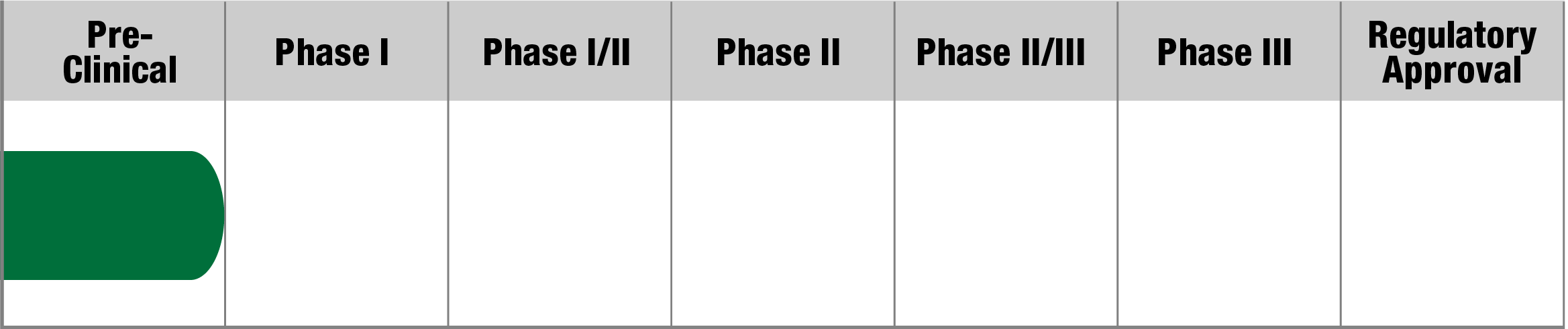
LHON
LUMEVOQ (GS010)
GenSight Biologics
Study Name:
REFLECT
Status:
Study active but not recruiting
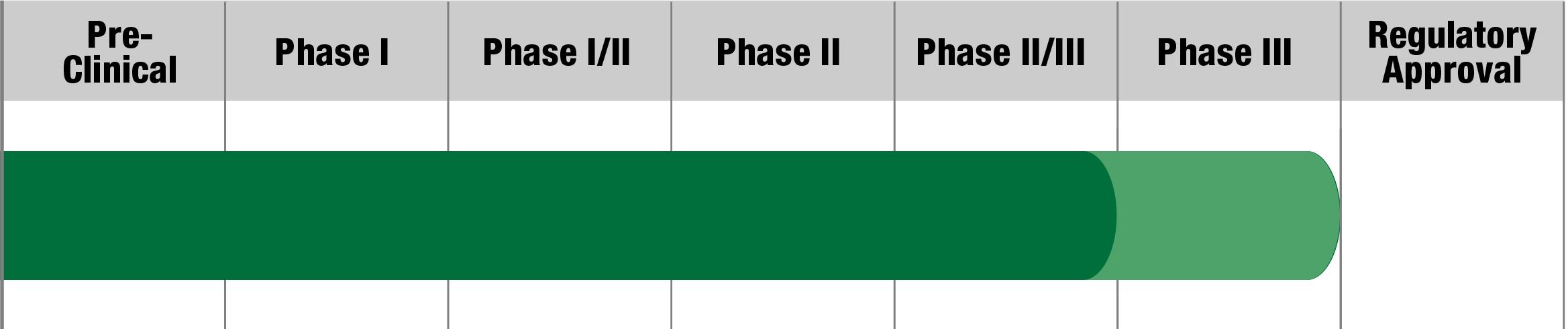
NR082
Neurophth Biotechnology
Status:
Actively Recruiting
(Beijing, China)
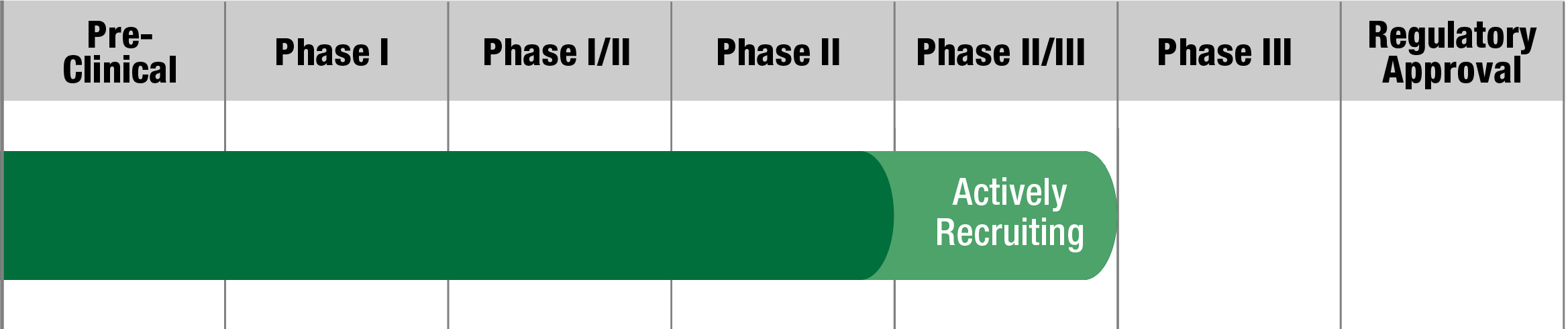
NFS-02
Neurophth Biotechnology
Status:
Paused
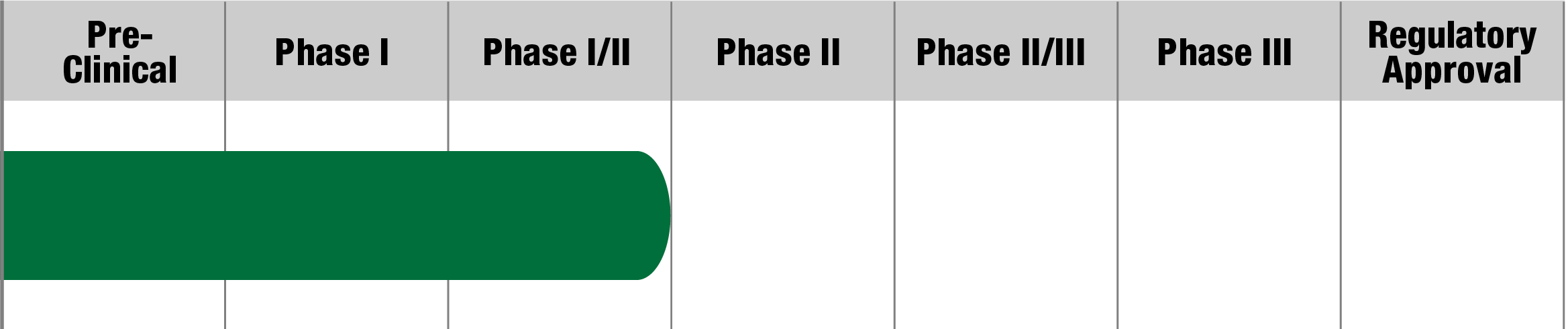
NR082
Neurophth Biotechnology
Status:
Actively Recruiting
(Sites: Palo Alto, CA;
Aurora, CO; Philadelphia, PA)
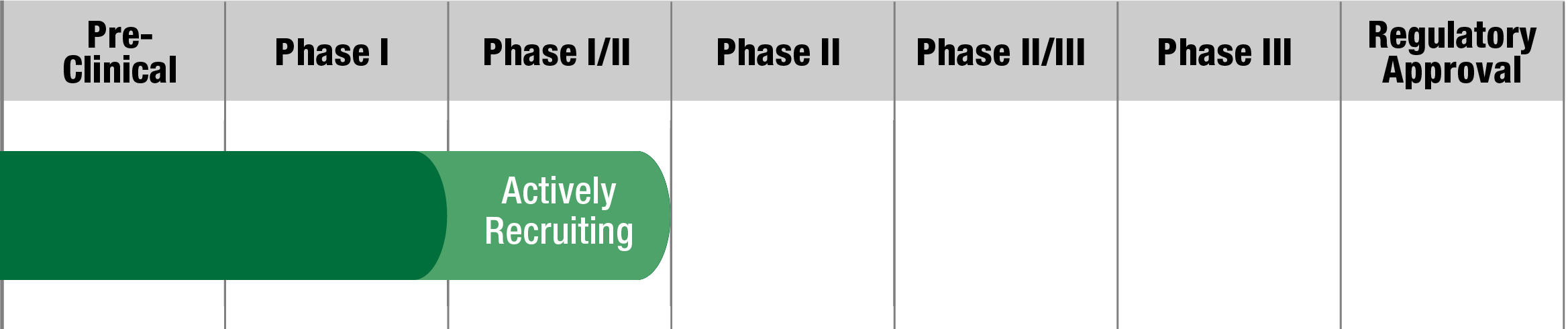
MELAS
OMT-28
Omeicos Therapeutics
Study Name:
PMD-OPTION
Status:
Fully Recruited

Zagociguat
Tisento Therapeutics
Study Name:
PRIZM
Status:
Actively Recruiting
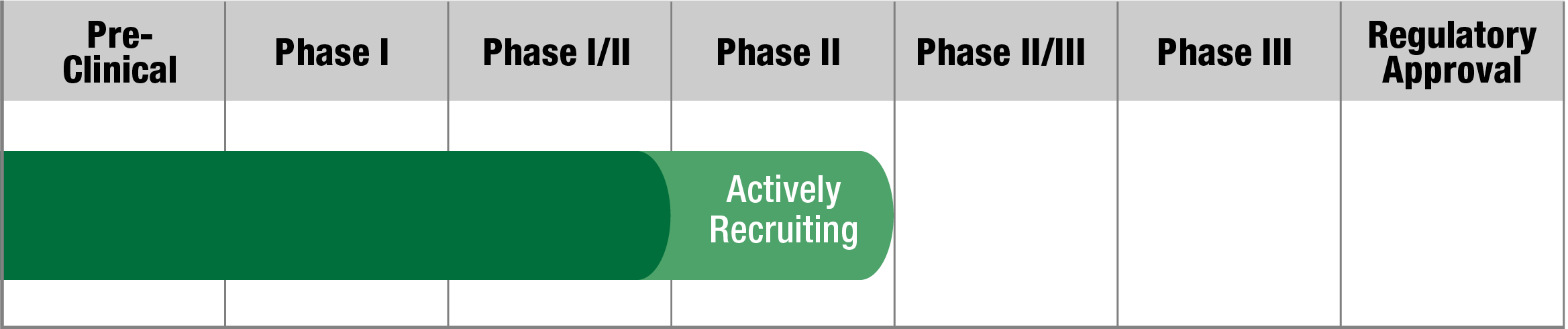
Sonlicromanol (KH176)
Khondrion
Study Name:
KHENEREXT
Status:
Fully Recruited

KL1333
Abliva AB
Study Name:
FALCON
Status:
Fully Recruited

MERRF
OMT-28
Omeicos Therapeutics
Study Name:
PMD-OPTION
Status:
Fully Recruited

KL1333
Abliva AB
Study Name:
FALCON
Status:
Fully Recruited

MIDD
OMT-28
Omeicos Therapeutics
Study Name:
PMD-OPTION
Status:
Fully Recruited

Sonlicromanol (KH176)
Khondrion
Study Name:
KHENEREXT
Status:
Fully Recruited

KL1333
Abliva AB
Study Name:
FALCON
Status:
Fully Recruited

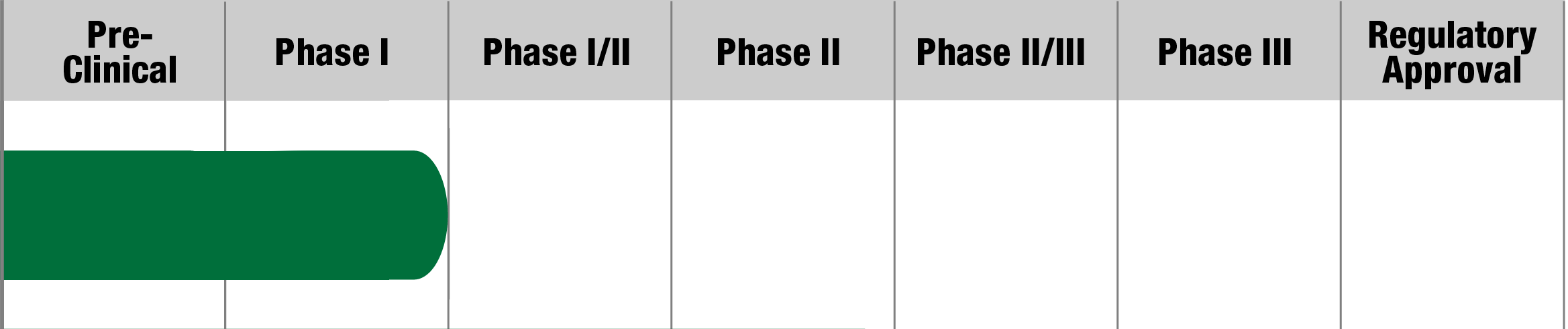
Pearson Syndrome
MNV-201
Minovia
Status:
Actively Recruiting
(Sites in Israel)
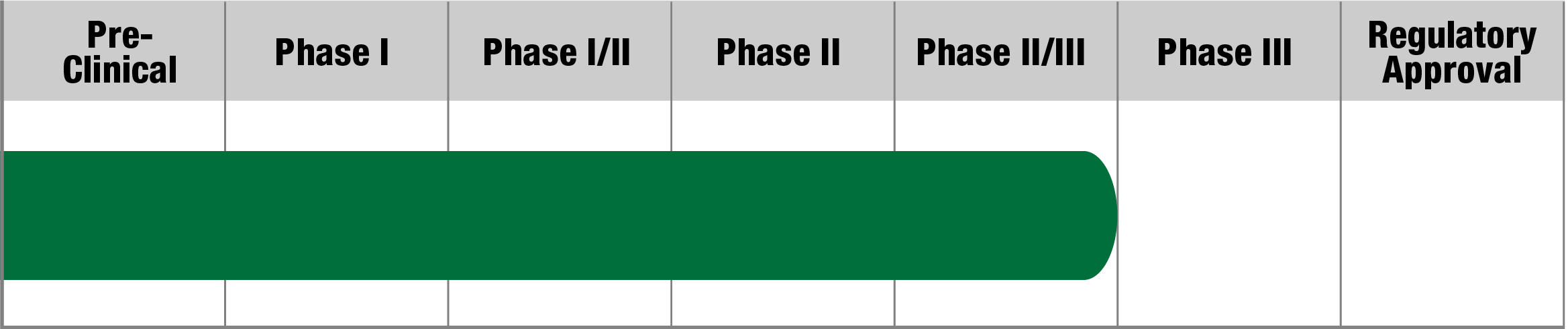
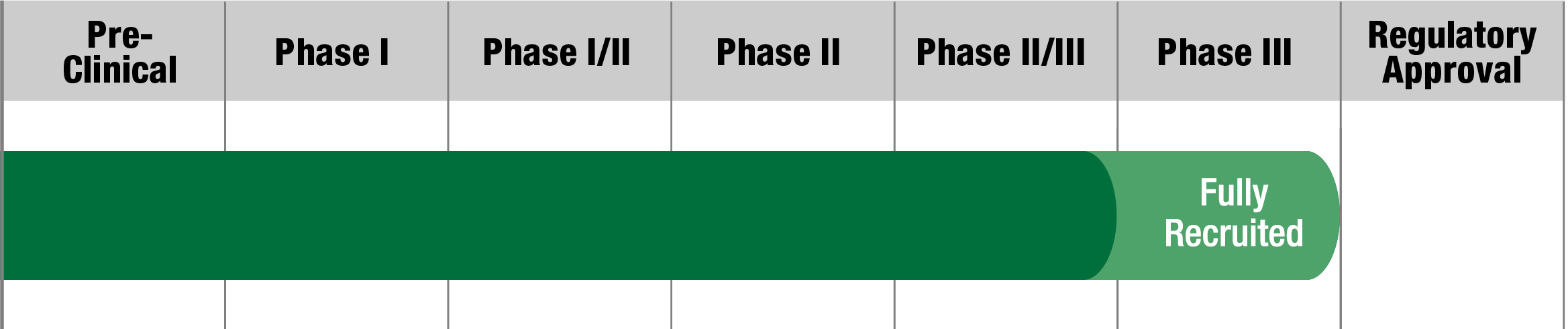
PDCD
SL-1009
(Sodium dichloroacetate)
Saol
Status:
Fully recruited
Primary Mitochondrial Myopathies (PMM)
Elamipretide (SS-31/MTP-131)
Stealth BioTherapeutics
Study Name:
NuPower
Status:
Fully Recruited

OMT-28
Omeicos Therapeutics
Study Name:
PMD-OPTION
Status:
Fully Recruited

TK2 Deficiency
MT1621
UCB
Status:
Open label study active, not recruiting